30+ Hydrochloric Acid And Sodium Hydroxide Equation
Hydrochloric Acid And Sodium Hydroxide Equation. Hydrofluoric acid + potassium hydroxide → chemistry. What happens when sodium hydroxide reacts with hydrochloric acid also write the chemical equation?

This net ionic equation is the same for the neutralization reaction of any strong acid and strong base. Sodium hydroxide hydrochloric acid balanced molecular equation complete and net ionic equation. Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (a salt) and water.
gris bleu peinture chambre grohe euphoria horse head vector png idealist
Reaction between Sodium hydroxide and nickel(ii)chloride
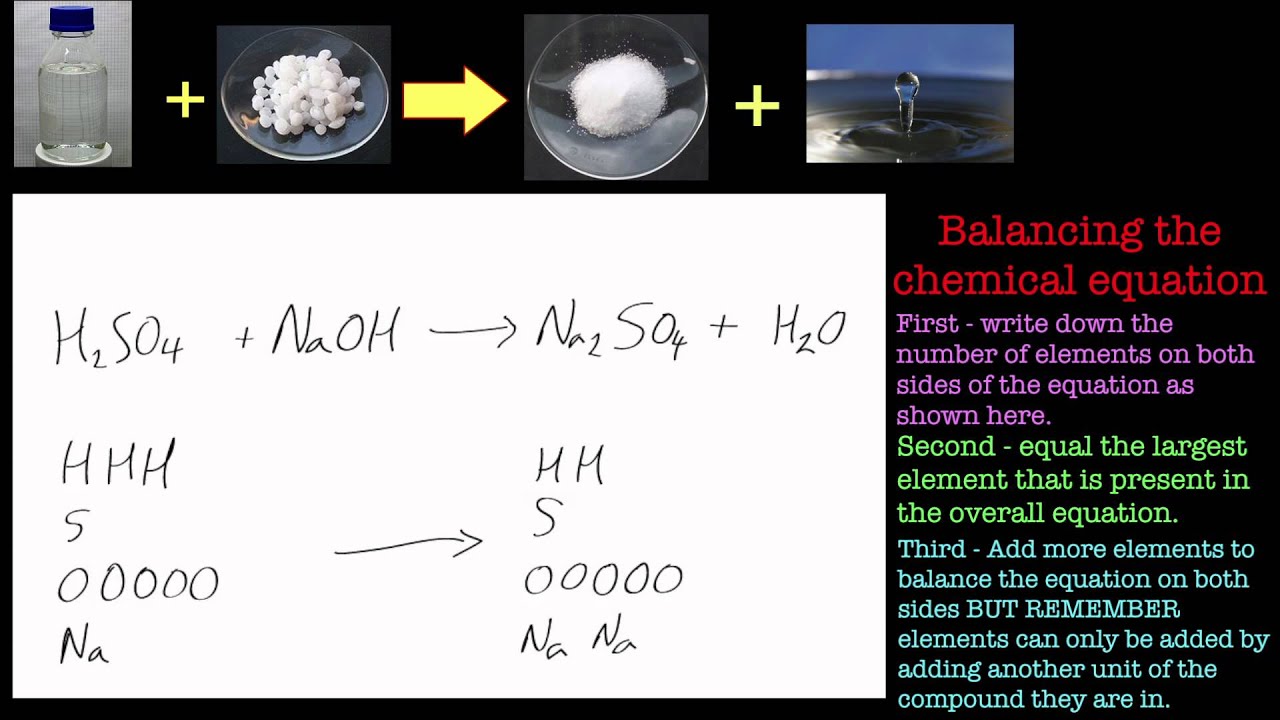
How to balance naoh hcl nacl h2o sodium hydroxide plus hydrochloric acid. The basic formulae hydrochloric acid ishcl and the basic formulae for sodium hydroxide is naoh so the balanced reaction between these two components is. When strong acids such as hcl (hydrochloric acid) reacts with strong base like naoh (sodium hydroxide), then neutral salt such as nacl (sodium chloride) and water are formed. Hcl + naoh = nacl + h₂o + q.

Sodium hydroxide hydrochloric acid balanced molecular equation complete and net ionic equation. Click to see full answer. Despite the aggression of the sodium hydroxide and hydrochloric acid, the reaction was a wonderful one. Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (the salt) and water. We need to combine sulfuric acid and sodium hydroxide to produce sodium sulfate.

What is the balanced equation for sulfuric acid and sodium hydroxide? Nitric acid + calcium hydroxide → 2. To balance naoh + hcl = nacl + h2o you'll need to be sure to count all of atoms on each side of the chemical equation. The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is: Sodium hydroxide.

Hcl (aq) + naoh (aq) → nacl (aq) + h 2 o (l) + heat. This net ionic equation is the same for the neutralization reaction of any strong acid and strong base. Sodium hydroxide + hydrochloric acid → sodium chloride + water the equation is balanced because no. Of atoms of each element in reactants is equal to no..

We need to combine sulfuric acid and sodium hydroxide to produce sodium sulfate and water. It also explains how to predict t. To write the ionic equation we must separate all aqueous species into their ions and leave any solid, liquid or gaseous substance in its molecular form. Click to rate this post! How to balance naoh hcl nacl h2o.

What is the balanced equation for sulfuric acid and sodium hydroxide? This leaves the ionic equation This is a titration problem. Hf (aq) + koh (aq) kf (aq) + h 2 o (l) molecular equation. Net ionic equation for the.

Once you know how many of each type. Click to see full answer. Hydrofluoric acid + potassium hydroxide → chemistry. It also explains how to predict t. Sodium hydroxide and hydrochloric acid net ionic equation see more:
Ionic equations a chemical equation shows the number of. Net ionic equation for the. Of atoms of each element of products. This leaves the ionic equation Hence, the correct answer is option (b).